 Home / Services / In Vitro Structural and Functional Characterization / In Vitro Kinase Screening & Profiling / Kinase Functional Assays / Colorimetric Assays for Kinase Activity
Home / Services / In Vitro Structural and Functional Characterization / In Vitro Kinase Screening & Profiling / Kinase Functional Assays / Colorimetric Assays for Kinase Activity Colorimetric Assays for Kinase Activity
INQUIRYCreative BioMart has developed a large collection of colorimetric assays to determine the activity of kinases for efficient kinase screening & profiling. Our scientific team with years of experience in kinase screening & profiling are able to provide unparalleled expertise to assist our customers in their kinase biology research and drug discovery.
Background
Kinases are one of the most extensively studied enzymes in biology and medical research. Kinases catalyze the transfer of phosphate from a donor ATP molecule to a wide variety of cellular substrates such as proteins, nucleic acids, lipids, and carbohydrates. Kinase activities are tightly regulated under normal physiological conditions. However, dysregulation of kinase activity under pathological conditions leads to many diseases such as cancer, cardiovascular diseases, neurological diseases, and inflammatory diseases. Consequently, kinases have become attractive therapeutic targets and technologies monitoring changes in kinase activity have emerged as important tools for drug development.
Kinase activity assays focus on directly or indirectly quantifying the production of catalytic products. Quantifying kinase activity requires detecting either the production of phosphorylated products or the amount of ATP used or ADP formed. Understanding the different types of kinase activity assays is a critical step for successful kinase research and drug discovery. Colorimetric assays have gained great interest due to their inherent advantages, including low cost, simple operation, adaptable sensitivity, quick response, wide linear range, and the possibility of qualitative or semiqualitative identification by the naked eye.
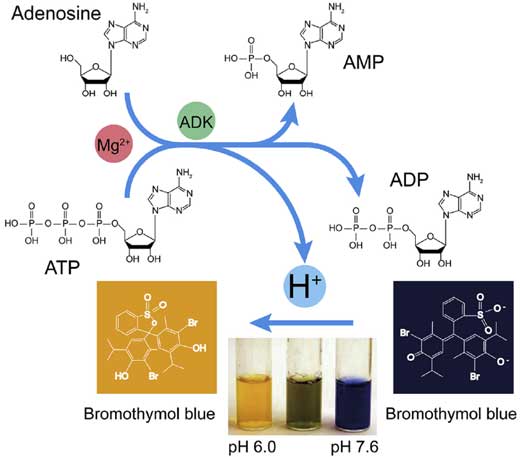
Figure 1. The schematic diagram of the assay for ADK activity. (Li Y, et al., 2018)
Featured Services
Creative BioMart offers custom colorimetric assays for kinase activity based on different methods to meet the specific needs of our customers. Our scientists will work with you to develop an optimum assay. If you can’t find an assay of your interest, please fill out the online inquiry form and let us know more details. We can offer you our expertise to help you accelerate your success.
Colorimetric ELISA
Different colorimetric assay formats have been reported for measuring kinase activities and screening inhibitors. Enzyme-linked immunosorbent assay (ELISA), which has the advantages of easy quantification, high specificity, high sensitivity, and high throughput, has become a powerful method for measuring protein phosphorylation. We have developed highly sensitive colorimetric biosensors for kinase activity detection by using ELISA to help our customers achieve desired results.
Nanomaterials-Based Colorimetric Assays
With the achievements of nanotechnology and nanoscience, nanomaterials-based colorimetric assays have emerged to be promising alternatives to conventional colorimetric ELISA. We have developed a wide range of colorimetric assay based on different types of nanomaterials, including gold nanoparticles (AuNPs), silver nanoparticles (AgNPs), palladium nanoparticles (Pd-NPs), magnetic nanoparticles (MNPs), carbon dots, etc. Our simple, robust, and universal assays enable our customers to make faster progress.
Creative BioMart is an advanced biotech company within the field of kinase/phosphatase biology. We offer our expertise in establishing assays for kinase screening & profiling. We have state-of-the-art facilities and technology to provide efficient services with guaranteed turnaround times. A large collection of ready-to-use kinase activity assay kits are also available to help our customers save precious time. If you have any questions, please do not hesitate to contact us.
References
- Li Y, et al. A rapid and sensitive colorimetric assay for the determination of adenosine kinase activity. Biochemical and biophysical research communications, 2018, 502(2): 250-254.
- Zhou J, et al. A gold nanoparticles colorimetric assay for label-free detection of protein kinase activity based on phosphorylation protection against exopeptidase cleavage. Biosensors and Bioelectronics, 2014, 53: 295-300.

